On March 24, press release No. 51/22 of the Court of Justice of the European Union was published in relation to the judgment in case C-533/20 Upfield Hungary, which concludes that “the list of ingredients of a food containing a vitamin does not have to mention the specific vitamin formula used. It is sufficient to include an indication of the name of the vitamin on the label of the food”.
Month: March 2022
Around the World – EU: Monitoring the presence of furan and alkylfurans in food
COMMISSION RECOMMENDATION (EU) 2022/495
- Member States should, with the active involvement of food business operators, monitor furan, 2-methylfuran and 3-methylfuran in food, in particular in coffee, jarred baby food (including baby food in containers, tubes and pouches), ready-to-eat soup, potato-based crisps, fruit juices, breakfast cereals, biscuits, crackers and crispbread.
- To ensure that the samples are representative, Member States should follow the sampling procedures laid down in part B of the Annex to Commission Regulation (EC) No 333/2007 Food business operators should also apply this sampling procedure or an equivalent sampling procedure, ensuring the sample is representative.
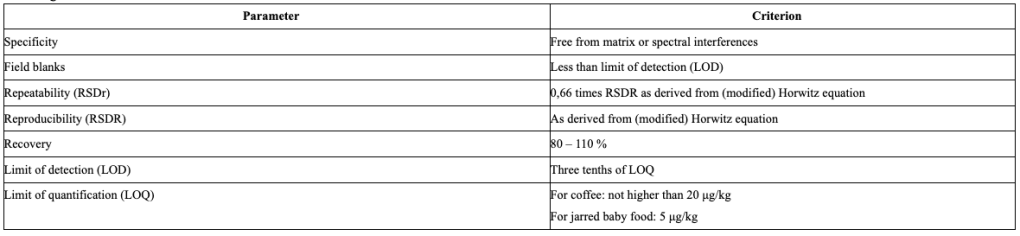
- For the analysis of furan, 2-methylfuran and 3-methylfuran in coffee and jarred baby food, Member States and food business operators should use a method that complies with the following criteria:
The Americas – Uruguay: National Bromatological Regulation 8th Edition 2022
The Americas – Colombia: The legal loopholes left by the regulation of cannabis for industrial use
According to Luz Helena Vargas, the first legal loophole has to do with CBD, “because although the authorities gave the green light to the use of THC products, CBD products are much more commercially attractive, a category for which will still be subject to the regulations issued by the Ministry of Health and Social Protection,” she explained.
Second, for now there is no regulation on the microbiological requirements for foods containing this ingredient (plant component, grain and non-psychoactive derivative of cannabis), therefore, “if an application is submitted at this time, it is not possible to start the procedures immediately, you have to wait until the ministry defines the specific requirements that these products must meet,” he said.

Around the World – EU: Draft Regulation on rules on conformity checks of marketing standards for olive oil and methods of analysis of the characteristics of olive
Draft Commission Implementing Regulation laying down rules on conformity checks of marketing standards for olive oil and methods of analysis of the characteristics of olive oil. This draft act includes provisions from the current EU legislation on olive oil standards (Regulation (EEC) No 2568/91 and Implementing Regulation (EU) No 29/2012) which relate to conformity checks carried out by EU Member States’ control authorities, collaboration on those checks, obligations of control authorities and operators as well as methods of analysis for determining the characteristics of olive oil.

