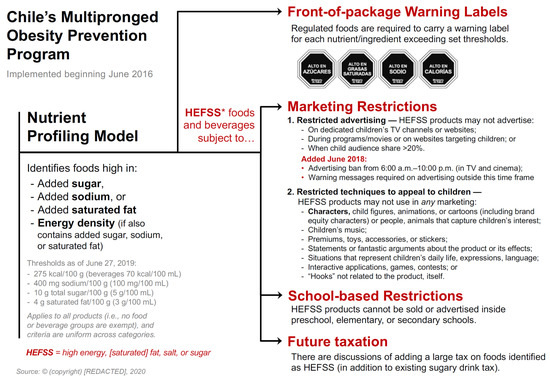
I analyse epistemic and non-epistemic factors involved in expert advisory practices and decision-making relating to the regulation of health claims in the US, EU, and Japan. I consider the changes that have taken place, historically, in regulatory policies in all three cases in order to confirm the hypothesis that not only epistemic, but also non-epistemic factors and values determine the methodological decisions on which expert assessments are based for the authorization of health claims. I found that the current European, US, and Japanese assessment systems are based on different kinds of what have been termed ‘epistemic policies’ and that these policies and other characteristics shape what might be identified as differing ‘policy styles’ in the regulation of health claims.