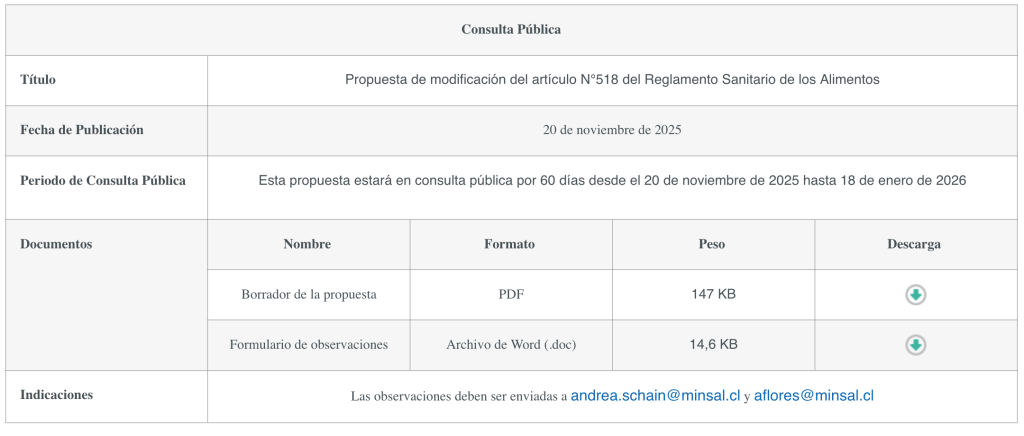
The Ministry of Health announces a public consultation to amend Article 518 of the Food Sanitary Regulations. The purpose of the proposed amendment is to stipulate that, for foods that have undergone fermentation and/or protein hydrolysis processes or that contain hydrolyzed ingredients, the limit for using the term “gluten-free” will be 10 ppm.
The health authority deems it necessary to amend Article 518 of the RSA, since gluten in hydrolyzed and fermented foods can only be quantified using the competitive ELISA method, which measures gluten at levels of 10 mg/kg or higher. This technique will not accurately determine gluten levels below 10 mg/kg with acceptable precision, and it will be impossible to meet the requirements set forth in the article. And if the sandwich ELISA technique were applied to these foods, small peptides would not be detected and the gluten content would be underestimated.
Article 518 of the RSA, since gluten in hydrolyzed foods as well as in fermented foods Fermented foods can only be quantified using the competitive ELISA method, but this method it quantifies gluten from 10 mg/kg. This technique will not determine gluten with acceptable accuracy gluten levels below 10 mg/kg cannot be accurately determined, and it will be impossible to meet the established requirements. in the article. And if the sandwich ELISA technique were applied to these foods, small peptides would not be detected and the gluten content would be underestimated. Small-sized peptides will not be detected, and the gluten content will be underestimated.