The Ecuadorian Standardization Service (INEN in Spanish) publishes in public consultation NTE INEN 2262 Second revision 2022 – ALCOHOLIC BEVERAGES. BEER. REQUIREMENTS.
Month: September 2022
Brazil – ANVISA develops new panel of additives of botanical species
The National Health Surveillance Agency (ANVISA in Portuguese) has made available for consultation the Panel of Flavoring Additives of Regional Botanical Species Approved for Use in Food. Through the tool you can consult the approved regional botanical species flavoring additives, the authorized use, the identification of the applicant and manufacturer and the instrument of approval.
Brazil – Food and packaging registration and post-registration amendment for public consultation
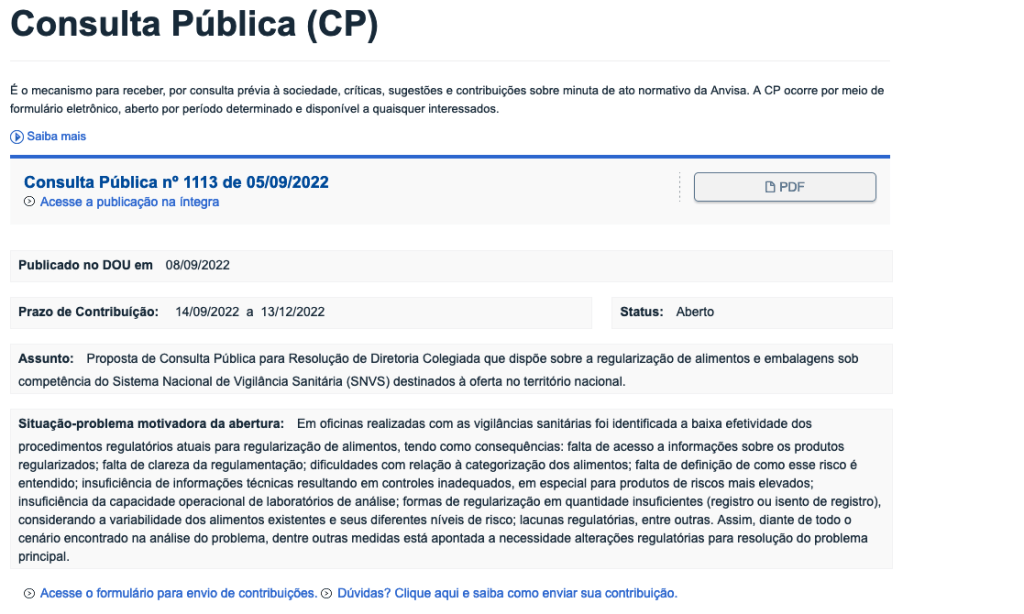
The National Health Surveillance Agency (ANVISA in Portuguese) opened the deadline to submit contributions to the Public Consultation (CP) 1113/2022, which deals with the proposed Resolution of the Collegiate Council of Administration (RDC) on the regularization of food and packaging under the competence of the National Health Surveillance System (SNVS). The proposal provides for changes in the registration and post-registration procedures, in addition to establishing notification for some categories, with a view to modernizing the regularization of these products. The deadline for comments and suggestions is December 13.
CP 1.113 is part of the revision process of RDC 22 and 23, both from 2000, which define the current regularization procedures for regulated food and packaging in the health area, and of RDC 27/2010, which provides for categories of exempted food and packaging and those requiring registration.
Guatemala – Update to the standard on health registration of dietary supplements published
The Ministry of Health published the update of Rule 14 version 2/2022 on sanitary registration of dietary supplements.
The purpose of this Technical Standard is to regulate the conditions and requirements through which the Department of Regulation and Control of Pharmaceutical and Related Products, hereinafter referred to as the Department, will grant the Sanitary Registration of dietary supplements.
Brazil – ANVISA performs regulatory impact analysis on food and packaging
The National Health Surveillance Agency (ANVISA in Portuguese) approved a Regulatory Impact Analysis report dealing with new procedures for the regulation of food and packaging. The decision was announced during the 16th Ordinary Meeting of the Agency’s Collegiate Board of Directors, within the scope of processes 25351.490309/2009-41 and 25351.490309/2009-41.
The overall objective of this update is to increase the effectiveness of the food and packaging regulation methods used by Anvisa and to ensure a more proportional treatment”.