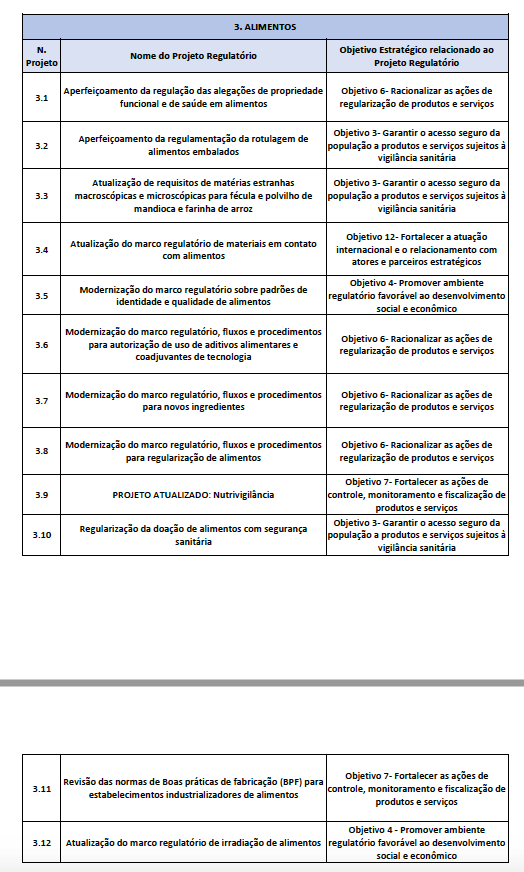
The National Health Surveillance Agency (ANVISA in Portuguese) released the Activities Report of the General Directorate of Food. The document presents a brief summary of the main results of the area in 2024, with emphasis on the risk and safety assessment processes, regulation of food and packaging, determination of regulatory and service standards, transparency and communication.
The relevant aspects in the report:
- Execution of 46% of the matters of the Regulatory Agenda 2024/2025 under its responsibility.
- Granting of two regulations in the matter with the Seal of Good Regulatory Practices, one in the gold category and another in the silver category.
- 46% reduction in global liability for registration, post-registration and evaluation applications.
- Greater agility in the analysis of requests for competence in the area, with a reduction in time of 30 days in the case of registration and post-registration and 55 days for risk and effectiveness assessments.
- Reduction in the percentage of rejections out of all approved requests, which reached the lowest level in the five-year historical series.
- Reduction in the response time of queries sent to the area by the Call Center, maintaining the percentage of user satisfaction (90%).