The National Service of Agricultural Health and Food Safety (SENASA in Spanish) has published the draft General Regulations on Agricultural Food Safety for public consultation. The General Regulations on Agricultural Food Safety aim to develop the provisions of Phytozoosanitary Law 157-94, amended by Decree 344-205, regarding food safety and suitability, in order to protect consumer health and facilitate food trade. The DIA’s agricultural food safety inspection and certification system must establish and implement regulatory programs and requirements for approval, inspection, certification, surveillance, and monitoring based on risk analysis concepts to ensure the supply of safe food. The scope of application covers the production of meat and meat products, fishery and aquaculture products, milk and dairy products, beekeeping products, fruits and vegetables, and animal feed in the primary production, processing, storage, import, and export phases.
Day: 21/05/2025
Venezuela – SENCAMER publishes 2 draft standards on mayonnaise-based sauces and pasteurized milk
The Decentralized Service for Standardization, Quality, Metrology and Technical Regulations (SENCAMER in Spanish) has published the following draft technical standards for public consultation:
- Draft Venezuelan Standard COVENIN 5040:2025 Mayonnaise-based sauces. Requirements.
- Draft Venezuelan Standard COVENIN 798:2025 Pasteurized milk. Requirements (4th Revision)
Argentina – ANMAT makes the purchase of imported food for specific medical purposes more flexible
By means of Provision 3280/2025, the National Administration of Medicines, Food and Medical Technology (ANMAT in Spanish) establishes:
The National Administration of Medicines, Food and Medical Technology (ANMAT) will not intervene in procedures related to direct user formalities such as the request for the proof of food for specific medical purposes of compassionate use, the request for authorization of entry of food for personal use and the authorization of entry of food from donations, repealed by ANMAT Provision No. 537/2025.
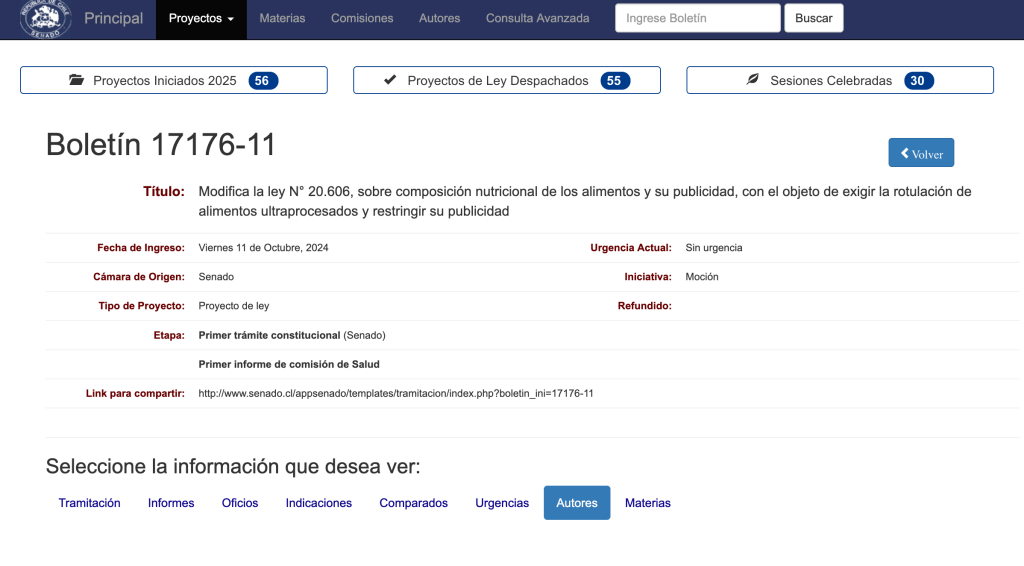
Chile – Senators propose a bill to amend Law No. 20,606 to incorporate the category “ultra-processed” in the stamp system
Senator María José Gatica presented the details of the bill that modifies Law N° 20.606 on the nutritional composition of food and its advertising, with the aim of requiring the labeling of ultra-processed food, restricting its advertising and contributing in this way to reduce diseases associated with overweight and obesity.
The National Renewal (RN) legislator explained that the proposal, supported by parliamentarians from different parties, seeks to incorporate the category of “ultra-processed” in the mandatory labeling of food. These products, linked to diseases such as obesity, hypertension and type 2 diabetes, would be identified with a specific seal on their label to provide clear and accessible information to consumers.
Together with Senator Juan Luis Castro, they agreed that “the current labeling system has been a world model, but it is necessary to move forward to include this type of food, which is directly related to the increase of chronic diseases in our society”.
Brazil – ANVISA publishes amendment on the regulation establishing the lists of components, limits of use, declarations and supplementary labeling of food supplements
The National Health Surveillance Agency (ANVISA in Portuguese) has published Normative Instruction IN No. 361/2025, which amends Normative Instruction IN No. 28/2018, which establishes the lists of components, limits of use, declarations and supplementary labeling of food supplements.
Amendments:
I – “List of components authorized for use in food supplements, except for food supplements intended for infants (0 to 12 months) or young children (1 to 3 years)”, contained in Annex I;
II – “List of minimum limits for nutrients, bioactive substances, enzymes, and probiotics that food supplements must provide, in the recommended daily intake and by population group indicated by the manufacturer”, contained in Annex III;
III – “List of maximum limits for nutrients, bioactive substances, enzymes, and probiotics that food supplements cannot exceed, in the recommended daily intake and by population group indicated by the manufacturer”, contained in Annex IV;
IV – “List of claims authorized for use on the labeling of food supplements and their respective composition and labeling requirements”, contained in Annex V; and
V – “List of requirements for the supplementary labeling of food supplements”, included in Annex VI.
Art. 2 The “List of components authorized for use in food supplements, except for food supplements intended for infants (0 to 12 months) or young children (1 to 3 years)” in Annex I of Regulatory Instruction – EN No. 28 of 2018, enters into force with the addition of the components listed in Annex I of this Regulatory Instruction.
Art. 3 The “List of minimum limits of nutrients, bioactive substances, enzymes, and probiotics that food supplements must provide in the recommended daily consumption and by population group indicated by the manufacturer” in Annex III of Regulatory Instruction – IN No. 28 of 2018, will enter into force with the addition of the limits listed in Annex II of this Regulatory Instruction.
Sole paragraph. Footnote iii is changed to “As cholecalciferol. 1 μg of cholecalciferol = 40 IU of vitamin D = 0.3333 μg of calcidiol.”
Art. 4 The “List of maximum limits of nutrients, bioactive substances, enzymes, and probiotics that cannot be exceeded by food supplements in the recommended daily intake and by population group indicated by the manufacturer” of Annex IV of Regulatory Instruction – IN No. 28, of 2018, will enter into force with the addition of the limits listed in Annex III of this Regulatory Instruction.
Sole paragraph. Footnote ii is changed to “As cholecalciferol. 1 μg of cholecalciferol = 40 IU of vitamin D = 0.3333 μg of calcidiol.”
Art. 5. The “List of Authorized Declarations for Use in the Labeling of Dietary Supplements and the Respective Composition and Labeling Requirements” in Annex V of Regulatory Instruction – IN No. 28 of 2018 will enter into force with the addition of the declarations listed in Annex IV of this Regulatory Instruction.
Art. 6. The “List of Supplementary Labeling Requirements for Food Supplements” in Annex VI of Regulatory Instruction – IN No. 28 of 2018 will enter into force with the incorporation of the supplementary labeling requirements listed in Annex V of this Regulatory Instruction.
Art. 7. A period of 24 (twenty-four) months is established for the adaptation of the labeling of food supplements that contain any of the constituents provided for in this Regulatory Instruction and that have been regularized before the National Health Surveillance System by the date of publication of this Regulatory Instruction.