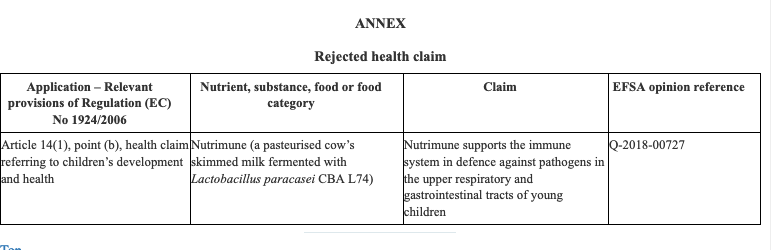
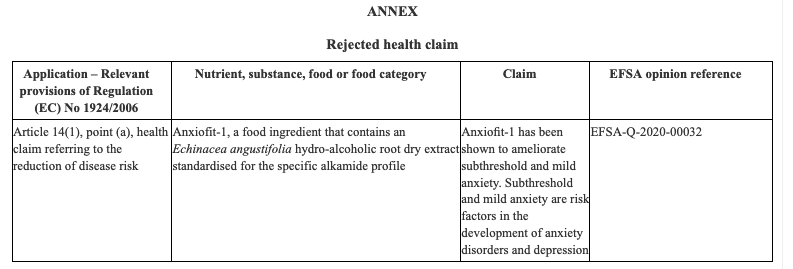
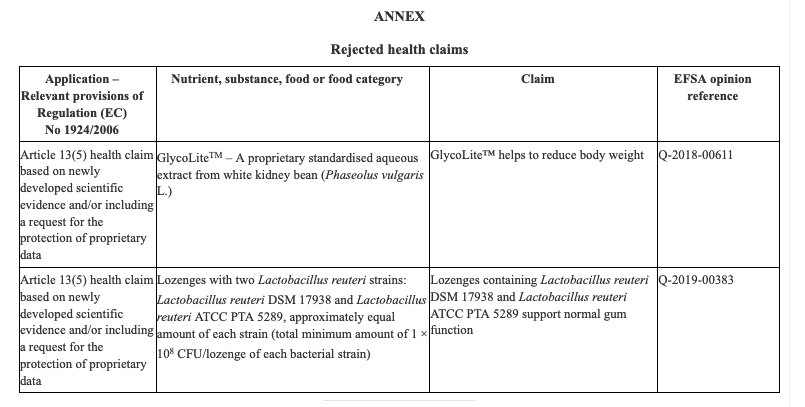
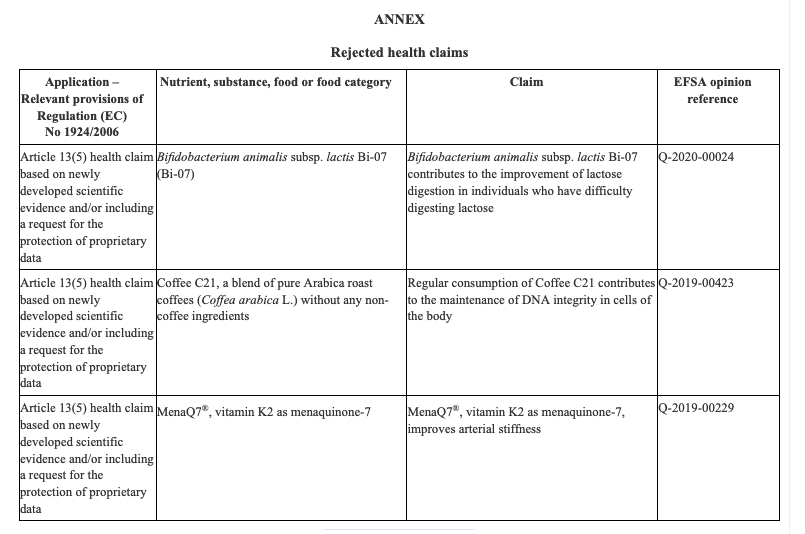
The Ministry of Agrarian Development and Irrigation (Midagri in Spanish) published Supreme Decree No. 006-2022-MIDAGRI that amends Article 27 of the Agri-Food Safety Regulation, which provides that primary agricultural foodstuffs must have more information through their labeling, documentation, technical data sheet or relevant information.
In the specific case of imported and domestic primary processed agricultural foods, the Spanish-language label shall contain the following information:
a. Name of the food.
b. Net content.
c. Country of origin or place of origin.
d. Name and address of the holder of the sanitary authorization.
e. Name and address of the importer, if applicable.
f. Sanitary Authorization of Primary Agricultural Food Processing Establishment.
g. Lot Identification.
h. Expiration Date or best before date.
i. Instructions for use and storage”.