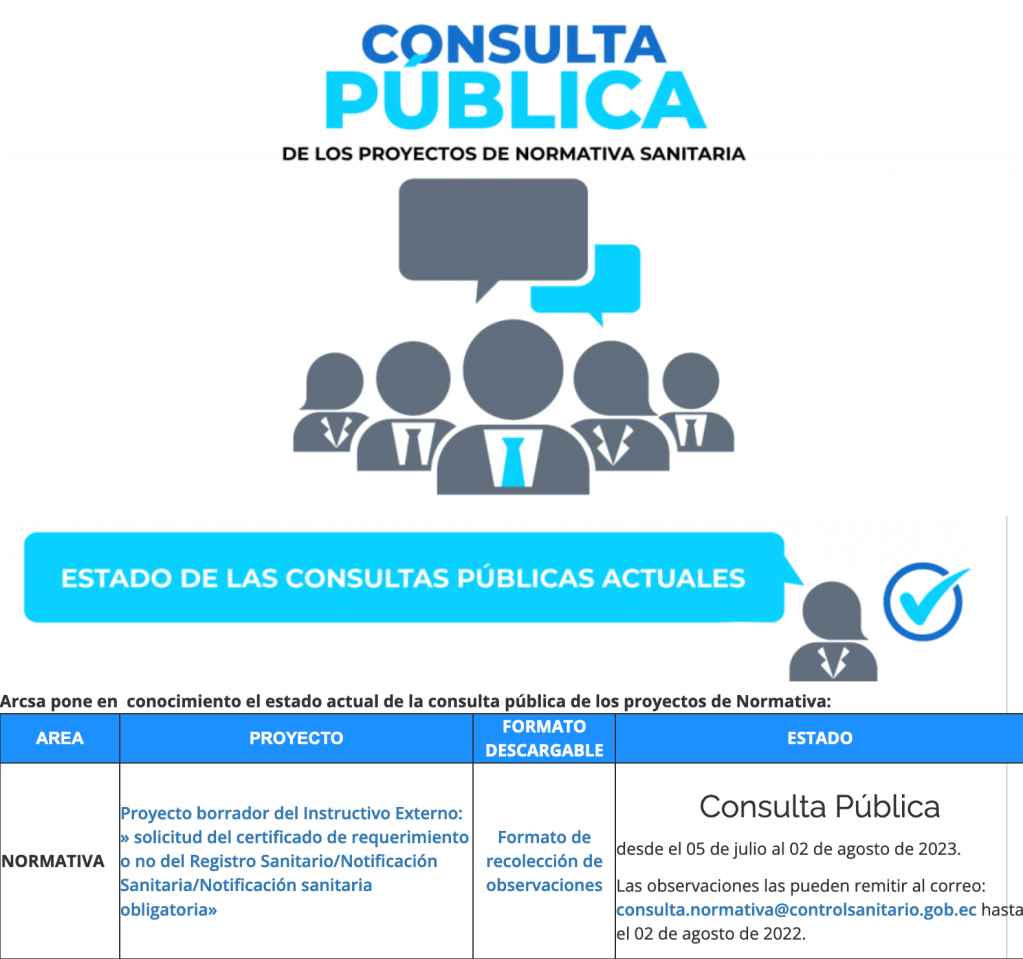
The National Directorate of Sanitary Surveillance (DINAVISA in Spanish) has issued two new Resolutions concerning the sanitary registration of food products, aimed at streamlining administrative processes, enhancing market access, and enabling the health authority to focus its regulatory resources on areas that significantly impact public health.
Resolution No. 326/2025
Establishes the requirement for every individual or legal entity to obtain the Establishment Registration (R.E.) prior to commencing any activities related to the manufacturing, importing, storing, or marketing of food products.
It introduces a classification of establishments based on their risk level (low, medium, and high), determined by the types of food they handle. Furthermore, it incorporates a system of sub-levels of risk assessed through a scoring table, which evaluates factors such as the nature of the establishment’s activities (manufacturer, importer, etc.), the implementation of food safety management systems (HACCP, ISO 22000), the degree of automation, and the establishment’s history.
This classification dictates the method and frequency of inspections, which now include modernized procedures:
On-site inspection: traditional in-person verification at the facilities.
Virtual/remote inspection: a distance procedure conducted via videoconference, requiring the establishment to have appropriate technology and connectivity.
Self-inspection: an assessment carried out by the company’s technical director, who swears under oath to comply with Good Practices (either Manufacturing or Storage).
The resolution also reinforces existing obligations, including that every individual or legal entity must possess a valid R.E. to request any Sanitary Registration of Food Product (R.S.P.A. in Spanish); the mandatory appointment of a professional technical director, and the inclusion of the R.E. number on product labeling.
Resolution No. 328/2025
This regulation outlines a risk classification, specifically focused on the final product. Applicants are required to assess the risk level of their products using the criteria and matrix defined in the resolution, which must subsequently be validated by DINAVISA. The regulation provides three registration pathways for obtaining the R.S.P.A.:
Automatic Health Notification (N.S.A.): applicable to low-risk products. Companies can obtain the R.S.P.A. immediately through DINAVISA’s electronic system (SIGRA) by submitting the required documentation under sworn declaration.
Simplified Health Notification (N.S.S.): intended for medium-risk foods. The application will be evaluated by DINAVISA within a maximum period of fifteen (15) days. If no resolution is reached within that timeframe, the registration will be automatically issued.
Ordinary Health Registration (R.S.O.): reserved for high-risk products. The evaluation process will last a maximum of thirty (30) days. Similarly, if there is no issuance within this period, the R.S.P.A. will be automatically granted.
Additionally, the new regulation stipulates that updates following the acquisition of registrations will be applied automatically, without the need for individual evaluation and approval procedures for each product.
Resolution No. 328/2025
This regulation outlines a risk classification, specifically focused on the final product. Applicants are required to assess the risk level of their products using the criteria and matrix defined in the resolution, which must subsequently be validated by DINAVISA. The regulation provides three registration pathways for obtaining the R.S.P.A.:
Automatic Health Notification (N.S.A. in Spanish): applicable to low-risk products. Companies can obtain the R.S.P.A. immediately through DINAVISA’s electronic Integrated Food Records Management and Control System (SIGRA in Spanish) by submitting the required documentation under sworn declaration.
Simplified Health Notification (N.S.S. in Spanish): intended for medium-risk foods. The application will be evaluated by DINAVISA within a maximum period of fifteen (15) days. If no resolution is reached within that timeframe, the registration will be automatically issued.
Ordinary Health Registration (R.S.O. in Spanish): reserved for high-risk products. The evaluation process will last a maximum of thirty (30) days. Similarly, if there is no issuance within this period, the R.S.P.A. will be automatically granted.
Additionally, the new regulation stipulates that updates following the acquisition of registrations will be applied automatically, without the need for individual evaluation and approval procedures for each product.