The Collegiate Council of the National Health Surveillance Agency (ANVISA in Portuguese) approved, during the 23rd Ordinary Public Assembly of the Agency, the update of the Regulatory Agenda 2024-2025. Based on the consolidated updates, the new list of regulatory topics of the Agenda for the 2024-2025 period now consists of 171 topics, distributed in 16 macro-themes of the Agency’s activities.
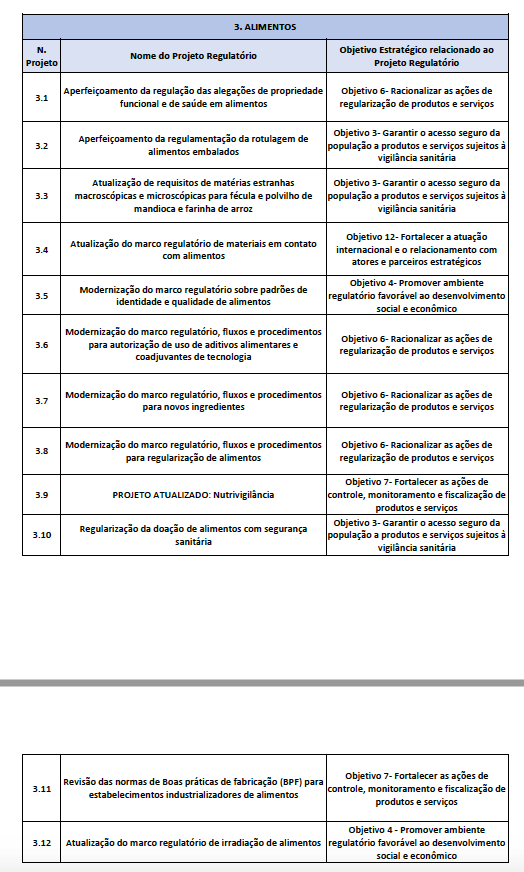
Of the 117 topics, 34 are food-related issues.
As foreseen in ANVISA’s Regulatory Agenda Manual, the set of prioritized topics can be reviewed annually, with the possibility of changes, exclusions and inclusions of topics.The objective of the annual update is to keep the document aligned with the regulatory priorities of the period, to ensure predictability and timeliness of the Agency’s regulatory planning.