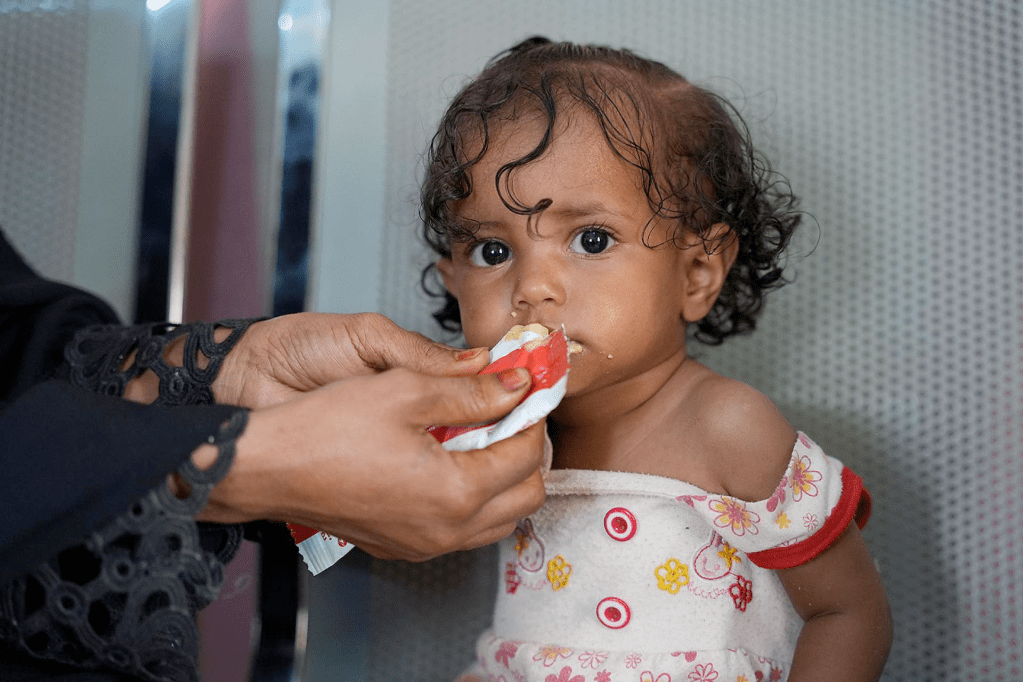
Codex guidelines for ready-to-use therapeutic food (RUTF) were adopted at the 45th session of the Codex Alimentarius Commission on 21 November and are set to pave the way for new innovative versions of the life-saving product. They will also enable governments to regulate safety effectively as manufacturers scale up production to tackle the malnutrition crisis affecting millions of children, especially in Africa and Asia.