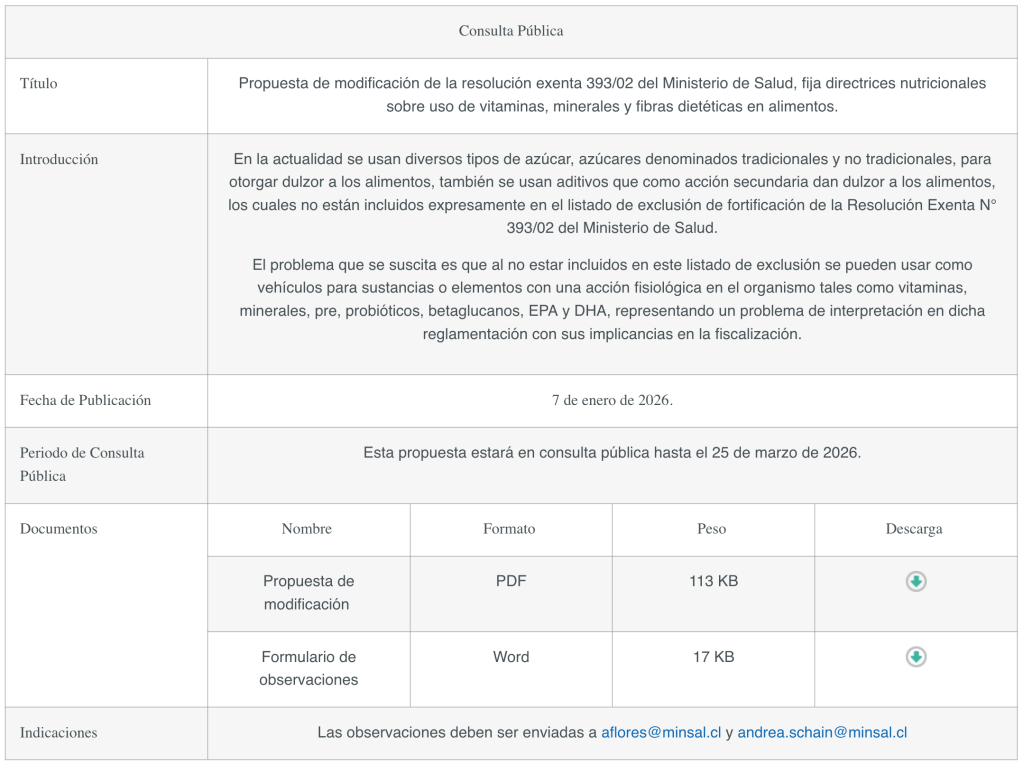
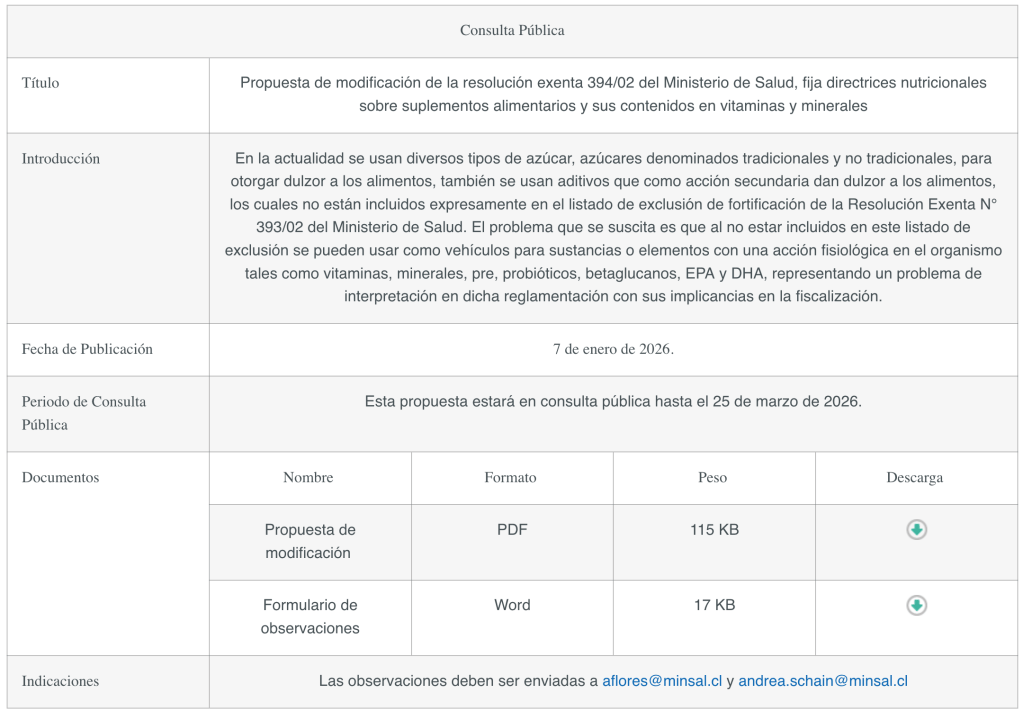
The Ministry of Health has published a proposed amendment to Exempt Resolution 393/02 of the Ministry of Health, which establishes nutritional guidelines on the use of vitamins, minerals, and dietary fiber in foods.
Currently, various types of sugar—both traditional and non-traditional—are used to sweeten foods, as are additives that, as a secondary effect, impart sweetness, none of which are expressly included in the fortification exclusion list of Exempt Resolution No. 393/02 issued by the Ministry of Health.
The problem that arises is that, since they are not included in this exclusion list, they can be used as vehicles for substances or elements with a physiological effect on the body, such as vitamins, minerals, pre- and probiotics, beta-glucans, EPA, and DHA, posing an interpretive challenge under the regulations and affecting enforcement.