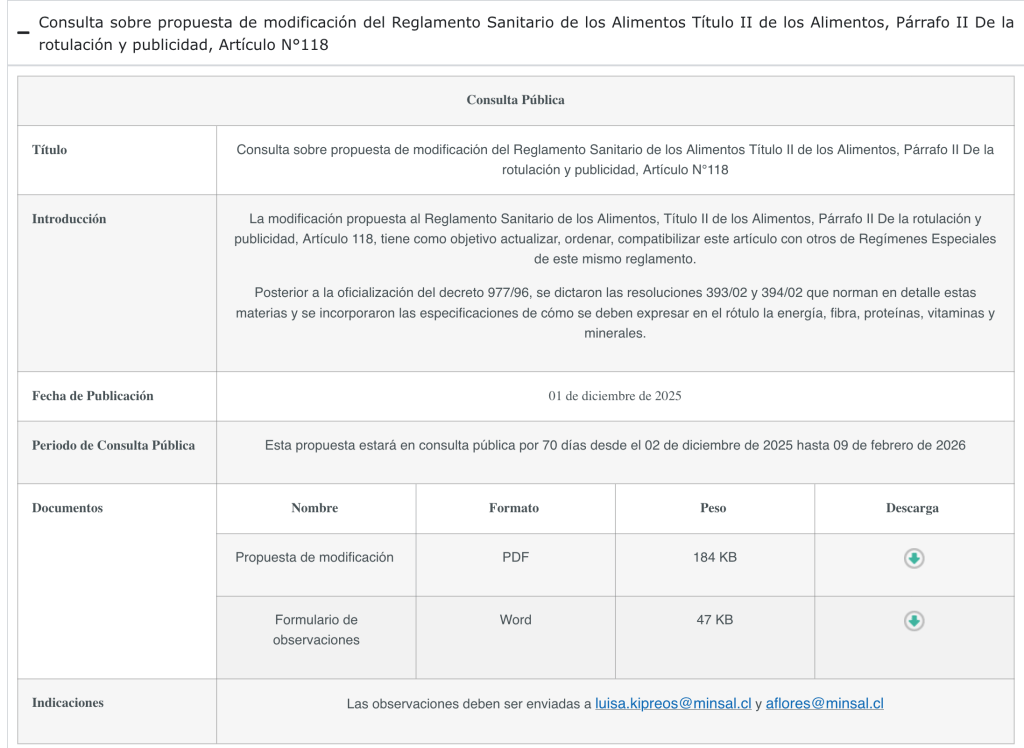
The Ministry of Health proposes amending the Food Sanitary Regulations, Title II on Foods, Paragraph II on Labeling and Advertising, Article 118, with the aim of updating, organizing, and aligning this article with other Special Regimes in the same regulations.
Following the official publication of Decree 977/96, Resolutions 393/02 and 394/02 were issued, which regulate these matters in detail and incorporate specifications on how energy, fiber, protein, vitamins, and minerals must be expressed on the label.
Proposed modification:
When a nutrient declaration is made, vitamins and minerals present in significant amounts—5% or more of the recommended intake for the population—may also be listed.
relevant. For the population over four years of age, the Daily Reference Intake (DRI) for energy, fiber, protein, vitamins, and minerals, as officially established by Exempt Resolutions 393/02 and 394/02, both issued by the Ministry of Health, or any future resolutions that replace or supplement them.
supplement.
For infants and children under four years of age, the ranges established in Title XXVIII, “Foods for Special Dietary Uses,” shall be used; for pregnant and breastfeeding women, the respective RDIs shall be used.
As Reference Daily Intakes, the respective RDIs shall be used. In the case of iron and vitamin A, the following will be accepted as the Daily Dose:
For iron and vitamin A, the reference daily intake during pregnancy is 30 mg/day for iron and 800 mcg/day for vitamin A. Numerical information on vitamins and the vitamin and mineral content must be presented in accordance with Article 115 of this Regulation, in metric units per 100 g or 100 ml, and per serving size, expressed as a percentage of the reference Recommended Daily Intake. Additionally, this information must be specified per serving on the label if the number of servings the package contains is indicated.