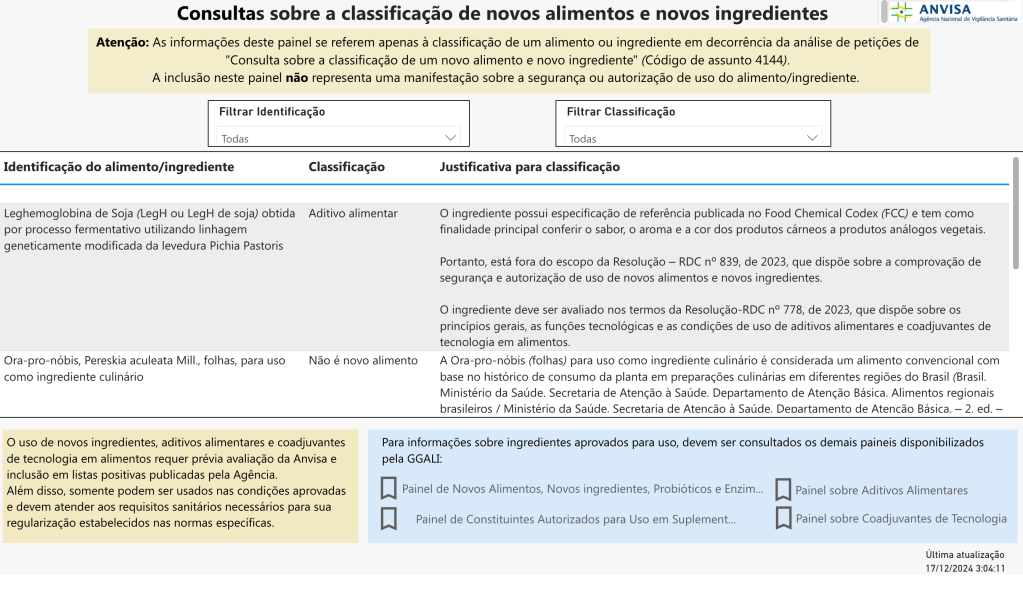
The National Health Surveillance Agency (ANVISA in Portuguese) announced the new panel on the classification of novel foods and novel ingredients. The Panel is the result of the implementation of regulatory modernization measures provided for in the Resolution of Collegiate Councils (RDC) No 839/2023, which provides for safety testing and authorization for the use of novel foods and novel ingredients. The results of consultations on the classification of a food or ingredient filed under the terms of RDC 839/2023 can be consulted in the tool. This is an optional procedure, available to provide more clarity on the classification of foods and ingredients. Interested companies can make this consultation through the Solicita System, using the request subject code 4144. Based on the information submitted, ANVISA’s technical team applies the regulatory definition of novel foods and novel ingredients to specific cases. However, inclusion in the panel does not mean a safety assessment or authorization to use these products.