The Department of Nutrition and Food, of the Division of Healthy Public Policies and Health Promotion of the Ministry of Health, published the draft modification of the “Message promoting healthy lifestyle habits in Food advertising” established in Decree N°1/2017 of the Ministry of Health.
Food or food products must include in their advertising carried out in mass media, a warning message, hereinafter referred to as the “Message”, when they have been “Message”, when sodium, sugars and saturated fats have been added to them, and content exceeds the value established in article 120 bis of the Supreme Decree No. 977 of 1996, of the Decree No. 977 of 1996, of the Ministry of Health, for those nutrients, or when they exceed the levels established for calories, sugars and saturated fats. when they exceed the levels established for calories and sugars or saturated fats have been added. sugars or saturated fats.
Month: August 2023
Uruguay – MERCOSUR technical regulation in public consultation
The Technological Laboratory of Uruguay (LATU) published the MERCOSUR Technical Regulations in public consultation.
- Draft Resolution Nº 02/23:
MERCOSUR technical regulation on definitions related to alcoholic beverages (except fermented beverages), their raw materials and manufacturing processes (Repeal of GMC Resolution Nº 77/94). - Draft Resolution Nº 03/23:
Modification of GMC Resolutions Nº 53/98, 54/98, 07/06 and 08/06 on food additives. - Draft Resolution Nº 04/23:
Modification of GMC Resolutions Nº 50/97, 53/98, 54/98, 16/00, 51/00, 08/06 and 09/07 on food additives. - Draft Resolution Nº 06/23:
Definition of contaminant (Modification of GMC Resolution Nº 31/92).

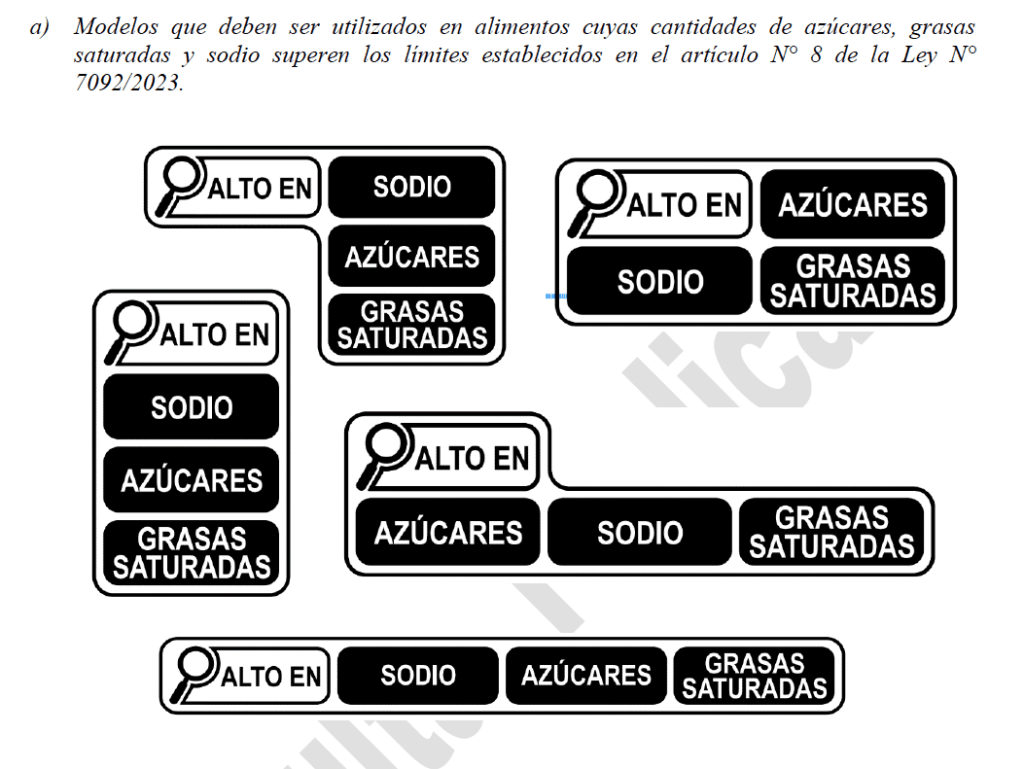
Paraguay – Public Consultation on Regulations of Law Nº 7092 on frontal warning labeling of packaged foods
The National Institute of Food and Nutrition (INAN in Spanish) presents to the public opinion, the industry and the productive sector in general, the Draft Decree of the Ministry of Public Health and Social Welfare, which regulates Law No. 7092 “on front warning labeling of packaged foods.
Argentina – Regulation of Law N° 27.669 on the regulatory framework for the development of the medical cannabis and industrial hemp industry published
Decree N°405/2023 approved the regulation of Law N° 27.669, on the regulatory framework for the development of the medical cannabis and industrial hemp industry.
The regulation establishes the creation of the National Agency of Hemp and Industrial Cannabis (ARICCAME in Spanish), which will be the regulatory body that will operate within the Ministry of Economy.
The main novelty of the regulatory framework is that it considers “psychoactive cannabis” those plants with dried flowers that exceed 1% of tetrahydrocannabinol (THC) in their chemical composition, therefore, up to that percentage it is legal from now on to manufacture products based on the substance, without entering into conflict with criminal legislation or international regulations and without having to go through the approval of the National Agency of Medicines (ANMAT in Spanish) through a new category, the so-called “vegetable”, for products that are not of medical grade.
As enforcement authority of the law, ARICCAME will define the specifications and regulation of what is considered a “product derived” from cannabis: human medicinal, veterinary, nutritional, cosmetic, industrial, plant health and fertility, but the regulation itself leaves the door open for new functionalities, “arising from scientific research and technological and industrial development”. For example, the seed as food or the oil extracted from it is rich in Omega 3, 6 and 9. And the fiber, as a textile material, can replace others that are pollutants.
All licenses will be valid for a minimum of five years and will be renewable. There will be seven types of license: hatchery, multiplication and cultivation; logistic services (transport, distribution, storage, packaging, among others); production of derivatives; commercialization of seeds, seedlings, cuttings and flowers; for studies and analytical tests; and foreign trade.

Brazil – 3rd edition of nutrition labeling Q&A guide
The National Health Surveillance Agency (ANVISA in Portuguese) published the 3rd edition of the Q&A guide on nutrition labeling. This edition brings a revision of some guidelines on added sugars, after the evaluation of doubts and requests received by the General Directorate of Food (GGALI in Portuguese) on the subject and the discussions of the Virtual Sectorial Dialogue on Added Sugars, held last July 3.