Introduction: Cereals are widely used in children’s nutrition. Objective: to evaluate the nutritional composition and labeling of cereal-based infant foods, in relation to current legislation. Material and Methods: cross-sectional, analytical and descriptive study that evaluated cereal-based foods, as well as labeling compliance with current Brazilian legislation. Results: 72 food samples were evaluated: cereal for baby food; mixture for the preparation of porridge and cereal flour. One hundred percent of the samples showed some non-compliance with the legislation, including the presence of a false concept of advantage and safety, illustrations not allowed, absence of mandatory warnings and, absence of the minimum age for consumption of the product. In bromatological and labeling analyses, the carbohydrate content of all categories exceeded 80% of the total energy value of the product. The protein, lipid, carbohydrate and energy contents of the cereal category for infant feeding showed significant differences, being, respectively, p=0.015, p<0.001, p=0.013 and p<0.001. The mix category for porridge preparation also showed significant differences for proteins, lipids, carbohydrates and energy (p<0.001). In the category of cereal flours, only the protein content showed a difference (p=0.05).
Conclusion: considering the sample universe of the study, it is possible to conclude that even in the presence of specific legislation, we still find legal non-conformities in the labeling of cereal-based foods intended for infant feeding, and these foods have a nutritional composition different from the information presented on their labels, negatively impacting children’s food safety.
Month: August 2023
Canada – Guidance document: Supplemented Foods Regulations
This guidance document is intended for stakeholders, including manufacturers and distributors of foods for sale in Canada, to facilitate the understanding of the Supplemented Foods Regulations, which came into force on July 21, 2022.
The Supplemented Foods Regulations should be read in conjunction with other provisions of the FDR applicable to pre-packaged products as well as the FDA and the documents incorporated by reference into the FDR (List of Permitted Supplemental Ingredients, List of Permitted Supplemented Food Categories, Directory of Supplemented Food Facts Table Formats, and Directory of Supplemented Food Caution Identifier Specifications), listed in Appendix 1. To account for new requirements specific to SFs in the FDR, certain consequential amendments had to be made to existing provisions in the FDR (for example, expanding labelling provisions in Part B, Division 1 applicable to the Nutrition Facts table to include the Supplemented Food Facts table). This document does not elaborate on these consequential amendments. Health Canada’s webpage on Supplemented Foods provides information and resources related to the requirements for SFs.
It is the responsibility of manufacturers and distributors to comply with all applicable legislative and regulatory requirements. In case of a discrepancy between this guidance and the provisions of the FDR or documents incorporated by reference, the regulations and the documents incorporated by reference take precedence.
In this guidance document, “must” is used to express a requirement, that is, a provision of the FDR that the manufacturer or distributor is obliged to satisfy; “should” is used to express a recommendation or that which is advised but not required; and “may” is used to express an option or that which is permissible within the limits of this document.

Costa Rica – Food labeling bill introduced in the legislature
Representative Andrea Álvarez of the National Liberation Party, presented bill No. 23861, for front labeling with nutritional warnings on food products and non-alcoholic beverages. According to the legislator, the project will label with octagons the products high in fats, sugars, calories, sodium or others. Products that do not exceed the levels already established worldwide based on scientific studies, would not have these seals that alert the consumer about the contents of critical nutrients.
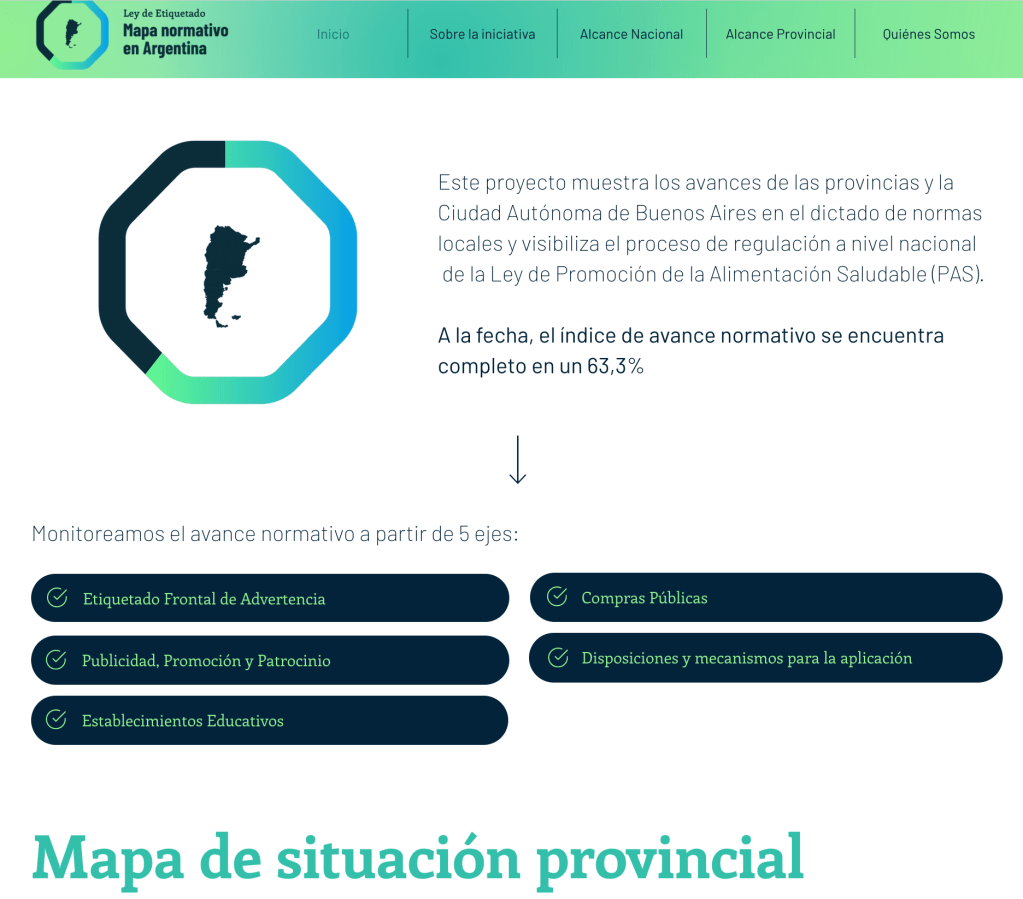
Argentina – Progress made by the provinces and the Autonomous City of Buenos Aires on the national regulation process of the Law for the Promotion of Healthy Eating (PAS)
This project shows graphically the progress of the 23 provinces and the Autonomous City of Buenos Aires (CABA in Spanish) in the enactment of local regulations governing the implementation of the Law on Promotion of Healthy Eating (PAS in Spanish) in the jurisdictions, and makes visible the process of regulation at the national level. It seeks to be a tool to promote and strengthen the full implementation of the law in all territories of Argentina.
Brazil – Technical Regulation on standards of identity and quality of dairy beverages is under public consultation
The Ministry of Agriculture and Livestock (MAPA in Portuguese) published ORDINANCE SDA/MAP No. 857 on public consultation of the Technical Regulation, which establishes the identity and quality standards to be met by dairy beverages. For the purposes of this regulation, dairy beverage is a dairy product, the composite dairy product, obtained from milk, reconstituted milk or dairy derivatives, or a combination thereof, with or without the addition of dairy ingredients.