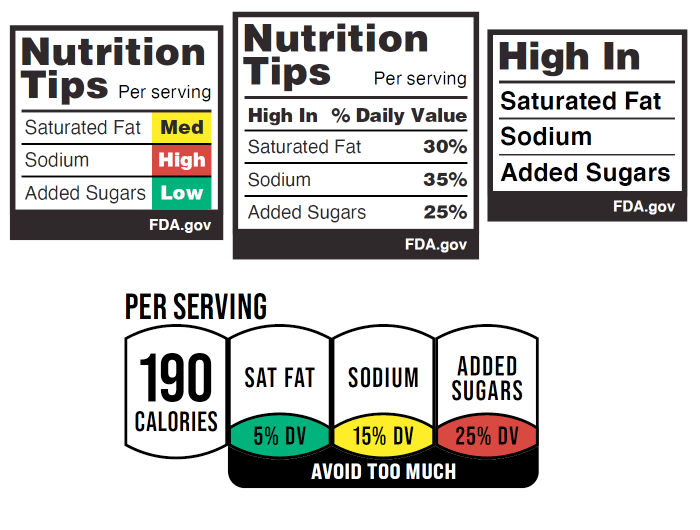
Momentum for mandatory front-of-package nutrition labeling continues to grow as new organizations are joining the effort to encourage the Food and Drug Administration to develop labels that would call attention to high levels of added sugars, sodium, and saturated fat in packaged, processed foods. And a new poll commissioned by CSPI finds strong public support for the proposal.
The American Cancer Society Cancer Action Network, American Heart Association, American Public Health Association, Consumer Federation of America, and Consumer Reports are among 17 organizations that have filed a supportive comment with the FDA, which opened a regulatory docket in response to an August 2022 petition filed by the Center for Science in the Public Interest, the Association of State SNAP Nutrition Education Administrators, and the Association of State Public Health Nutritionists.