Commission Implementing Regulation (EU) 2022/672 of 22 April 2022 amending Implementing Regulation (EU) 2017/2470 as regards the specifications of the novel food trans-resveratrol.

Commission Implementing Regulation (EU) 2022/672 of 22 April 2022 amending Implementing Regulation (EU) 2017/2470 as regards the specifications of the novel food trans-resveratrol.

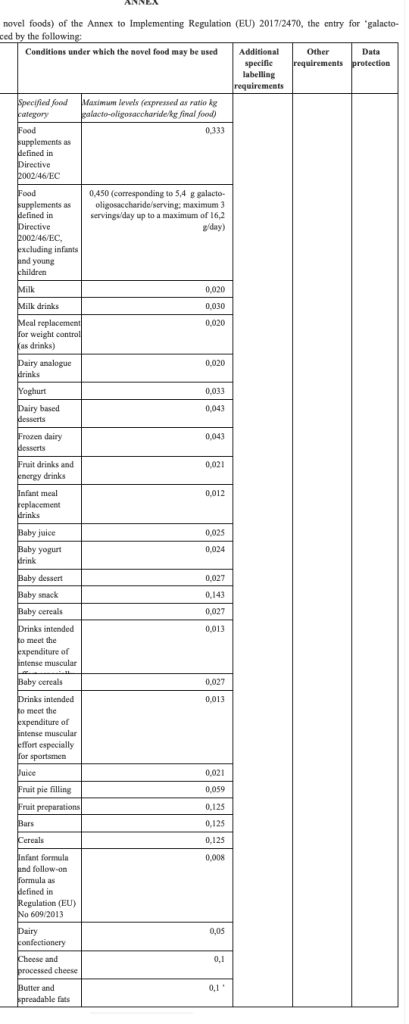
Commission Implementing Regulation (EU) 2022/684 of 28 April 2022 amending Implementing Regulation (EU) 2017/2470 as regards the conditions of use of the novel food galacto-oligosaccharide
The Ministry of Health opened a public call to begin a study, with the “best available evidence”, to identify what type of front warning seal a package with high levels of sodium, sugars or saturated fats should have.
The tender is titled “Front labeling process” and requests the evaluation of the best available evidence to establish shapes, color, size, legends and location of the front warning labeling for processed products in Colombia. In the study, two reports must be submitted: the first one, 15 days after the bid is submitted, on how the study will be carried out; the second one, the complete results by July 17, 2022.

The initiative, which has been approved by the Senate, creates the regulatory framework for the production and national commercialization for export of the cannabis plant, its seeds and derived products for medicinal use, including scientific research and industrial use, promoting the development of the sector’s production chain.
In addition, a regulatory agency, the Agencia Regulatoria del Cáñamo y del Cannabis Medicinal (ARICCAME in Spanish), will be created. It will regulate the import, export, cultivation, industrial production, manufacture, commercialization and acquisition of seeds, the cannabis plant and its derivative products for medicinal or industrial purposes.
