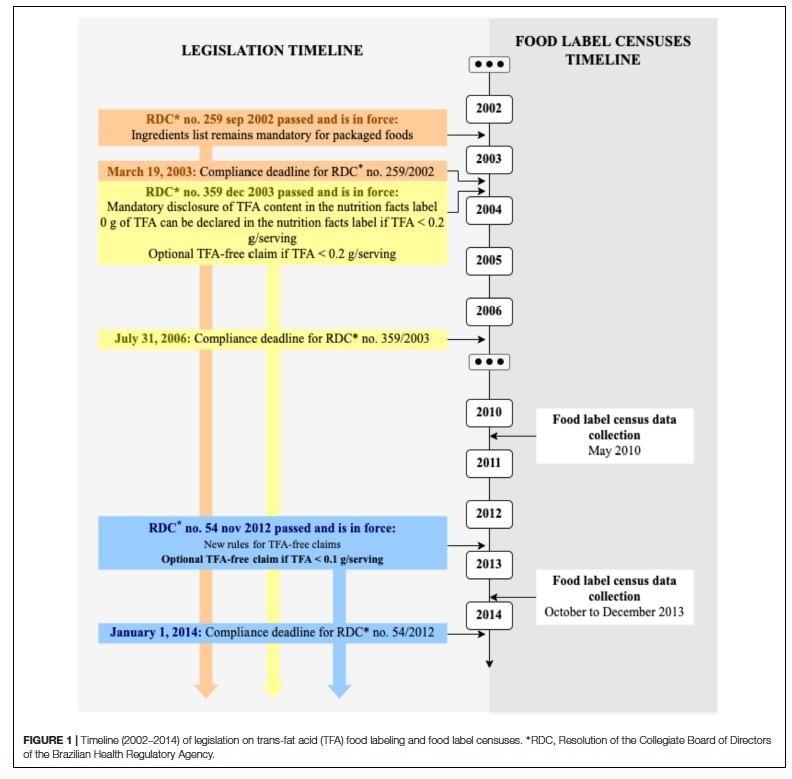
The consumption of food supplements has increased considerably, especially whey protein. Since many users make their choices through the product label, it is important that this information may be transparent for good understanding. For this, there are responsible actors for overseeing whether the information is in accordance with the standard and thus offering safe and quality products to consumers. This study aimed to evaluate the labelling adequacy of whey protein food supplements sold in Fortaleza, in the state of Ceará/BR. A checklist based on Collegiate Board Resolution number 243/2018 was applied to 51 supplement labels, obtained from five stores specializing in nutritional products, thus excluding products with manufacturing data prior to July 2018 and with a foreign language without translation. The identifying labels were obtained from 29 different brands, 25 (92%) of which were domestically manufactured and four (8%) were imported. Non-conformities were found in the layout related parameters and the characters appearance with respect to the product designation, such as background color contrast in the label, designation readability, location of the product designation, boldface designation and designation complemented with the nutrition font from which it was extracted and the method of use. In addition, many filtered products did not contain information about conservation after opening the package. Regarding the product designation “food supplement”, only two (4%) products were suitable. The findings of this study showed that the labelling adequacy of whey protein supplements was not adequate to current legislation and that the industry will take time to adapt its labels to the new resolutions.