 Yury Caldera
Yury Caldera
After 60 years of being rejected, three years of being scientifically reviewed by the WHO, and two years of diplomatic discussions, last December 2nd, 2020, the UN recognized the therapeutic properties of the cannabis flower. The multilateral organization decided to eliminate cannabis for medical use out of the list of the 4th Convention on Drugs of 1961.
This progress has made it possible to decriminalize it to be able to use it therapeutically in different Latin American countries and at the same time, the extraordinary industrial versatility of the non-psychoactive hemp has been identified that consists of oils, resins, dyes, crude extracts, or other innovations resulting from technological development derived from the non-psychoactive cannabis or hemp, with a content of THC less than 1%, including cannabinoids, isomers, acids, terpenes, salts, and salts of isomers, derived from plant material such as stems, seeds, seed hulls, woody material, or other foliar material such as biomass.
Nowadays, the use of derivatives as a raw material in the food industry is of great interest (see Image 1), once it’s been confirmed that hemp seeds and their derivatives (such as oil and flour) contain: Polyunsaturated fatty acids (PUFAs) with low concentrations of saturated fatty acids; proteins with an exceptional content of amino acids that contain sulfur, which is methionine, cysteine, and arginine, in addition to its content of vitamins and minerals, which provides it with a great nutritional value and utility in the food industry.
Recognizing its potentialities to use it in food and supplements is currently under discussion, considering that being products for human consumption, the national sanitary authorities have a special interest and they must be regulated. In this regard, Latin America has shown important progress in this matter. Countries like Colombia, Ecuador, Uruguay, Chile, and Paraguay, (Chart 1) have made some progress in different levels, and others like Argentina, are working on regulations to authorize the industrial use of hemp in foods, beverages, and dietary supplements.
Chart 1. Regulatory progress in the industrial use of non-psychoactive hemp in food
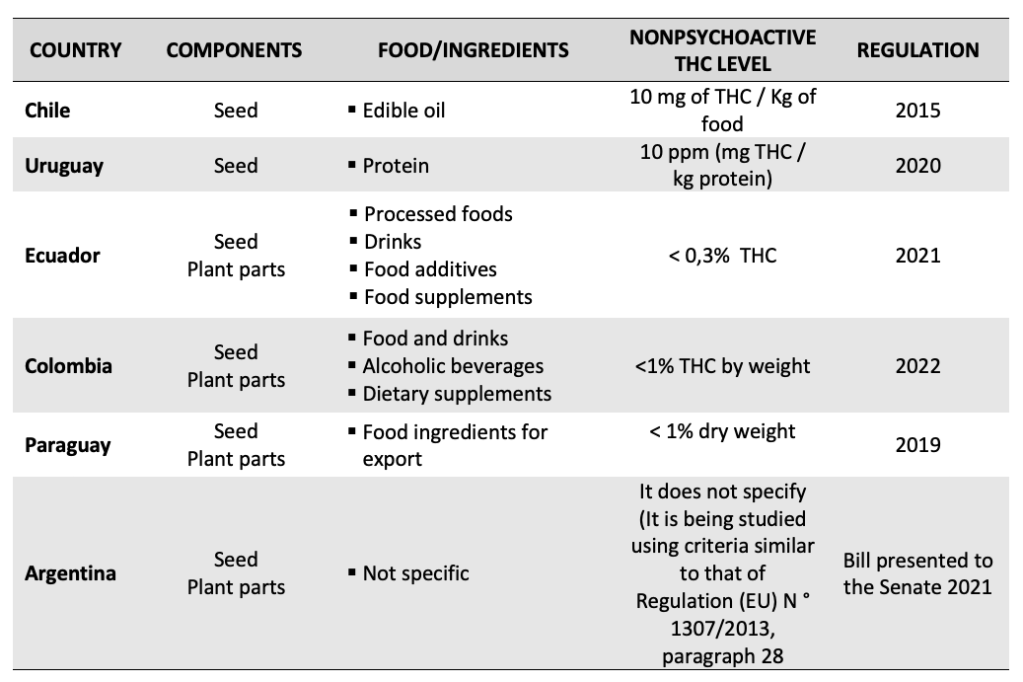
The following are the most relevant aspects:
Chile
Since 2015, through Exempt Resolution N° 432, they have authorized getting edible oil derived from Hemp seeds of cannabis sativa. However, it specifies it cannot contain Tetrahydrocannabinol (THC). In 2016 a public consult notice was sent to the World Trade Organization to modify article 170 of the Food General Regulations that would establish the maximum level of Tetrahydrocannabinol (THC) in human nutrition and hemp-based foods (Cannabis sativa), which should be 10 mg of THC/Kg of food. It was used as a reference to the maximum level in Canada and EFSA background. The suggestion hasn’t been yet included in the national law.
Uruguay
In January 2020, Decree 19/020 was published; it modified the National Bromatological Regulation to include the protein of the hemp seed that results from the seeds or nuts of the industrial hemp as a food ingredient. The hemp protein will be processed in powder form since this is the ingredient’s presentation admitting a THC concentration of up to 10 ppm (mg THC/kg protein) for the powdered ingredient. This decree describes the composition of macronutrients in % and also the expected characteristics of the amino acids. In addition, it takes into account that all the labels of the products should show the statement “Protein of the hemp seed” in the list of the ingredients.
Ecuador
In February 2021, Resolution 002-2021 on “Regulation and control of finished products of human use and consumption containing non-psychoactive cannabis or hemp” was published. The regulation authorized all the parts of the non-psychoactive cannabis or hemp as a food ingredient, as well as the non-psychoactive cannabis or hemp derivatives whose values are less than 0.3% of THC in industrial food, beverages, food additives, and dietary supplements. In addition, it establishes the requirements for labeling, advertising, and the express prohibition to use the claims relating to the health benefits.
Colombia
In July 2021, Decree 811 was published, substituting title 11 “On the safe and informed access to the use of cannabis and cannabis plant”. The new regulation stipulates that for industrial purposes, it will be taken into account the following uses other than medical and scientific, including, but not limited to food, beverages, and dietary supplements. The use of grain, plant components, and products derived from non-psychoactive cannabis is authorized. In any case, those products for industrial purposes derived from cannabis and/or plant components whose content of Tetrahydrocannabinol (THC) including its isomers, salts, and acid forms is less than one percent (1 %) in weight.
In February 2022, the regulation of Decree 811 was published, through Resolution 227, which describes the requirements that must be met by food, beverages, and dietary supplements that include non-psychoactive cannabis derivatives as ingredients.
Paraguay
In October 2019, Resolution N° 829 came into force. It authorizes the non-psychoactive industrial hemp farming for its industrial use, and established that it is decriminalized when it’s “non-psychoactive cannabis” containing “THC of less than 1% in dry weight”. This progress makes it possible for the country to position itself as the leader in exporting oil, proteins, and industrial hemp derivatives, and it became the first Latin American country to export non-psychoactive cannabis food derivatives to the European Union and the United Kingdom. However, this is a paradox due to the fact that the national regulation does not authorize its use in the food category.
Argentina
In June 2021, the Senate passed a bill on the “Regulatory framework for the development of the medical cannabis and industrial hemp industry”. One of its goals is to legalize different productive links and the hemp or industrial hemp commercialization that includes the food category and its sub-products, as well as promoting the authorization mechanisms for the producers and distributors, and safety, control, and traceability strategies in the chain.
Conclusions
Currently, they’re still working on a phase of discovery and development globally to improve or create new products, ways to commercialize, and regulatory modalities to be applied according to the categories of sanitary interest like food. However, the progress they’ve made so far will continue and more and more countries in Latin America will incorporate new regulations and amendments that allow the industrial use of hemp in food safety, with quality and efficiency by consumers.
References
- Decree No. 811, which replaces title 11 “On safe and informed access to the use of cannabis and cannabis plant”. Ministry of Social Protection (July 23, 2021). Link
- Decree No. 19-2020, Modification to the National Bromatological Regulation. Products for Food Use. Hemp Seed Protein. Link
- López, A. (2021). The cannabis value chain: international situation and trends, and opportunities for Argentina. Documentos de Trabajo del CCE N° 1, March 2021, Consejo para el Cambio Estructural – Ministerio de Desarrollo Productivo de la Nación. Link
- Ministry of Health of the Republic of Argentina. (2021). National government presented the project to regulate the development of the medical cannabis and industrial hemp industry (Official News, June 22, 2021). Link
- Chile Notification G/SPS/N/CHL/536. Committee on Sanitary and Phytosanitary Measures of the World Trade Organization, 2016. New Article 170 bis of the Food Sanitary Regulations, Supreme Decree No. 977/96 of the Ministry of Health. Link
- Pineda Ereño D. Global regulatory trends in the use of CBD in foods and food supplements. Regulatory Focus. Published online June 30, 2021.Link
- Draft regulatory framework law for the development of the medical cannabis and industrial hemp industry. NO-2021-53823924-APN-SSAP#JGM. (Filed June 15, 2021). Link
- Exempt Resolution No 432 of July 29, 2021 of the Ministry of Health, published in the Official Gazette of 08.08.15 “Authorizes the obtaining of edible oil from the seeds of Hemp Cannabis Sativa.. Link
- Food Sanitary Regulation. Decree NO. 977/96. (D.OF. 13.05.97). Updated February 2, 2021. Link
- Resolution 227/2022. Resolution 227/2022. By which Decree 811 of 2021 is regulated, which replaces Title 11 of Part 8 of Part 8 of Book 2 of Decree 780 of 2016, in relation to licenses, quotas and authorizations for the safe and informed access to the use of cannabis and the cannabis plant, its derivatives and products, and establishes other provisions”. safe and informed access to the use of cannabis and the cannabis plant, its derivatives and products, and other provisions are established.” Link
- Resolution No. 839. “Whereby the guidelines for the importation of non-psychoactive industrial hemp (cannabis sp.) are established; and SENAVE Resolution No. 631/19, dated September 09, 2019, is extended, within the framework of the provisions of Decree No. 2725/19 dated October 21, 2019”. Link
- Santi Carneri. BBC Planeta Futuro. The original article was produced with the support of the Gabo Foundation grant for “New Narratives on Drugs in Latin America”. Link
- Resolution ARCSA-DE-002-2021-MAFG. Technical Sanitary Regulations for the Regulation and Control of Finished Products for Human Use and Consumption Containing Non Psychoactive Cannabis or Hemp, or Derivatives of Non Psychoactive Cannabis or Hemp. Link
- World Health Organization. Recommendations by the WHO Expert Committee on Drug Dependence (ECDD) on the appropriate scheduling of psychoactive substances within the international drug conventions [letter, WHO director-general to UN secretary-general]. Organization. Link